Many remote Aboriginal and Torres Strait Islander communities in Australia experience unacceptable high rates of STIs such as chlamydia, gonorrhoea and trichomonas, despite efforts over many years to bring these infections under control.
A key public health strategy for control of curable STIs is the provision of accurate testing and timely treatment through primary care services.
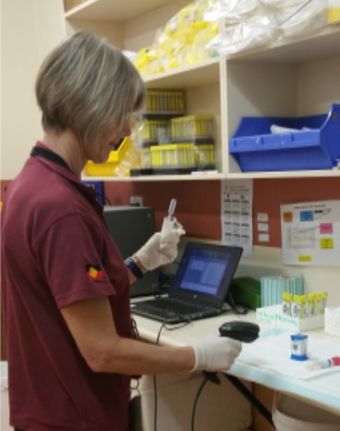
Recent technological advances have led to the development of highly accurate point-of-care (POC) tests for STIs using the GeneXpert machine, based on nucleic acid amplification technology (NAAT).
The TTANGO (Test Treat ANd GO) research trial was carried out from 2011-2016 to determine the acceptability, performance and short term health impact of this new POC technology for the detection of chlamydia (CT) and gonorrhoea (NG) in remote communities.
The TTANGO Trial Final Report findings demonstrated:
- Stakeholders, clinical staff and clients indicated POC testing for STIs was highly acceptable in remote and regional settings and made suggestions for future programs
- The GeneXpert CT/NG test used by clinical staff at the point-of-care in these settings was as accurate as conventional laboratory-based tests for CT and NG
- POC testing and use of these results to guide clinical management was associated with more timely treatment, reducing delays on average by 9 days
TTANGO2
Building on the TTANGO Trial, TTANGO2 was a translational research program, funded by an NHMRC Partnership Grant and the Australian Government Department of Health, which focused on the wider implementation of STI POC testing in high prevalence areas in regional and remote Australia.
Through TTANGO2, STI POC testing for chlamydia, gonorrhoea and trichomonas on the Cepheid GeneXpert® was undertaken in a network of 32 primary health services over a 5-year period to evaluate the long-term uptake, sustainability and impact of STI POC testing in these services.
TTANGO3
With continued funding through the Australian Government Department of Health, TTANGO is now in a routine program phase (TTANGO3) with more services being enrolled, expanding to up to 80 services by June 2024.
STI POC testing, as well as enhanced continuous quality improvement practices, has led to earlier detection and treatment uptake for many Aboriginal and Torres Strait Islander people. The TGA-approved Xpert® CT/NG and Xpert® TV test cartridges for use on the GeneXpert platform enable the detection of chlamydia and gonorrhoea infections in 90 minutes, and trichomonas infections in 60 minutes, respectively